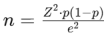Abstract
This study investigates the public health risks associated with veterinary drug residues in animal products within selected areas of Oromia, Ethiopia, a region heavily reliant on livestock for economic sustenance and food security. Utilizing a cross-sectional survey methodology, the research involved 209 livestock producers from both urban and rural settings to evaluate their awareness regarding the presence and risks of drug residues in food products, as well as their adherence to prescribed withdrawal periods following veterinary drug administration. The findings reveal alarming knowledge gaps, with a significant portion of respondents (63.1% to 70.2%) unaware of the potential health hazards linked to drug residues in animal-derived food products. Additionally, only 25.8% of farmers reported that they consider withdrawal periods before marketing their livestock or livestock products, indicating a critical lapse in food safety practices. These lapses not only jeopardize public health but also threaten the integrity of the livestock sector and its contribution to the economy. Given these findings, the study underscores the urgent need for improved regulatory frameworks, enhanced educational outreach, and increased access to veterinary services. By implementing targeted interventions aimed at raising awareness and compliance with withdrawal periods, stakeholders can significantly mitigate the risks associated with veterinary drug residues. This research highlights the importance of collaborative efforts among government bodies, veterinary professionals, and livestock producers to ensure safer animal husbandry practices and protect public health in Oromia, Ethiopia.
|
Published in
|
Animal and Veterinary Sciences (Volume 13, Issue 1)
|
|
DOI
|
10.11648/j.avs.20251301.11
|
|
Page(s)
|
1-6 |
|
Creative Commons
|

This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited.
|
|
Copyright
|
Copyright © The Author(s), 2025. Published by Science Publishing Group
|
Keywords
Veterinary Drug Residues, Food Safety, Antibiotic Resistance, Livestock, Oromia, Withdrawal Periods
1. Introduction
The growing use of veterinary drugs in livestock production has raised significant concerns about the safety of food derived from treated animals. Residues from veterinary antibiotics and other pharmaceuticals can persist in animal products such as meat, milk, and eggs, posing risks to consumers and public health. These residues can cause allergic reactions, disrupt gut microbiota, and contribute to the development of antibiotic-resistant bacteria, which is a global public health crisis. Studies have consistently reported antimicrobial residues in food products, including tetracyclines, sulfonamides, and fluoroquinolones in milk, meat, and eggs, exceeding recommended safety limits in some cases
| [3] | Beyene, T. (2016). "Veterinary drug residue management and food safety in Ethiopia: Challenges and opportunities." International Journal of Veterinary Science |
| [14] | Treiber, F. M., & Beranek-Knauer, H. (2021). "Antibiotics and food safety: Residues of tetracyclines, sulfonamides, and fluoroquinolones in food products." Antibiotics, 10(5), 534. https://doi.org/10.3390/antibiotics10050534 |
[3, 14]
.
Research highlights the persistence of these residues even after standard cooking or processing, with implications for food safety and nutrition. For example, quinolones and sulfonamides demonstrate high thermal stability, making them particularly concerning for consumer health
| [5] | Food Science of Animal Resources. (2019). "Thermal stability of quinolones and sulfonamides in food products and implications for consumer safety." Food Science of Animal Resources, 39(6), e58. https://doi.org/10.5851/kosfa.2019.e58 |
[5]
. Ensuring compliance with regulatory maximum residue limits (MRLs) is critical to safeguarding public health and reducing the risk of long-term antibiotic exposure through the food chain
.
In Ethiopia, veterinary drug residues in food products remain a significant concern, particularly in rural areas where livestock farming is a critical livelihood. Studies have highlighted that poor regulation, lack of awareness among farmers, and non-compliance with drug withdrawal periods contribute significantly to contamination issues. For instance, antimicrobial residues such as tetracyclines and sulfonamides have been detected in meat and dairy products at levels exceeding safety limits, posing public health risks like allergic reactions and antibiotic resistance
| [3] | Beyene, T. (2016). "Veterinary drug residue management and food safety in Ethiopia: Challenges and opportunities." International Journal of Veterinary Science |
[3]
.
The challenges are amplified by limited access to veterinary services and unregulated drug distribution systems in rural Ethiopia. Farmers often lack knowledge about withdrawal periods, with studies reporting non-compliance rates of 100% among surveyed farmers in some regions. This ignorance exacerbates the risks of drug residues in animal products entering the food chain
.
Addressing veterinary drug residue issues in Ethiopia requires a multi-faceted approach, including comprehensive farmer education, stricter enforcement of withdrawal periods, and enhanced residue-monitoring systems. Such measures are critical for safeguarding food safety, reducing public health risks, and supporting the agricultural economy, which heavily relies on livestock for domestic consumption and export.
Research highlights the importance of improved veterinary practices and regular testing programs to mitigate residue contamination in food products. These efforts align with advancements in residue detection technologies and strategies to ensure compliance with regulatory standards, ultimately promoting consumer confidence and international trade opportunities
| [4] | Beyene, T., & Tesega, B. (2014). "Rational veterinary drug use: Its significance in food safety, public health, and animal productivity." Journal of Veterinary Medicine and Animal Health, 6(12), 359-365. https://doi.org/10.5897/JVMAH2014.0332 |
| [15] | Wu, Q., et al. (2023). "Advancements in residue detection technologies and strategies for ensuring compliance with veterinary drug regulations." Sustainability, 15(13), 10413. |
[4, 15]
.
The research seeks to inform policy decisions and promote better practices among livestock producers, ultimately mitigating the risks associated with veterinary drug residues.
Objectives
1) To evaluate the levels of veterinary drug residues in various animal products within selected areas of Oromia, Ethiopia.
2) To assess the awareness and knowledge of livestock producers regarding the risks associated with veterinary drug residues in food.
3) To investigate the adherence of farmers to recommended withdrawal periods for veterinary drugs before selling animal products.
2. Materials and Methods
2.1. Study Area
This study was conducted in six districts of Oromia, Ethiopia: Bora and Adami Tulu Jido Kombolcha (East Showa), Kofale and Shashemane (West Arsi), and Digalu Tijo and Asella (Arsi Zone). These districts were selected due to their high levels of livestock production, diverse agricultural practices, and the presence of both urban and rural environments. The selection aims to provide a comprehensive understanding of the challenges faced by livestock producers concerning veterinary drug use and the awareness of potential residues in animal products.
2.2. Study Design and Population
A cross-sectional survey design was employed to facilitate the assessment of knowledge, practices, and food safety concerns among livestock producers. The target population comprised livestock producers in both urban and rural settings, ensuring a representative sample of the local farming community. A total of 209 livestock producers were selected using a stratified random sampling technique, which accounted for demographic variations in the study area, such as the type of livestock raised and socio-economic status.
2.3. Sample Size Determination
The sample size of 209 was determined using Cochran’s formula for sample size estimation, which is suitable for estimating proportions in a population. The formula is given by:
Where:
n = required sample size
Z = Z-value (1.96 for a 95% confidence level)
p = estimated proportion (0.5 for maximum variability)
e = margin of error (0.05)
Using this formula, the calculated sample size was adjusted to account for the population size and potential non-response, resulting in a final sample size of 209 participants.
2.4. Data Collection
Data collection was carried out using a structured questionnaire developed based on a thorough literature review and expert consultations. The questionnaire included multiple sections covering:
1) Demographic Information: Age, gender, educational level, and farming experience of respondents.
2) Veterinary Drug Use: Types of veterinary drugs used, sources of procurement, and adherence to prescribed dosages.
3) Awareness of Drug Residues: Knowledge of the risks associated with drug residues in animal products and sources of information.
4) Food Safety Practices: Practices concerning withdrawal periods for veterinary drugs, handling of livestock products, and preventive measures taken to ensure food safety.
The survey was administered through face-to-face interviews conducted by trained enumerators fluent in the local language. A pre-test of the questionnaire was conducted in a nearby district to ensure clarity, reliability, and validity before the actual data collection began.
2.5. Reliability and Validity Testing
The reliability of the questionnaire was assessed using Cronbach's alpha, a measure of internal consistency. A Cronbach’s alpha coefficient of 0.70 or higher was considered acceptable, indicating that the questionnaire items consistently measured the intended constructs.
The validity of the questionnaire was evaluated through expert reviews, where subject matter experts assessed the relevance and clarity of each item. Construct validity was further examined through exploratory factor analysis (EFA) to ensure that the items grouped as expected according to the theoretical constructs.
2.6. Data Analysis
Data were entered and analyzed using statistical software (SPSS). Descriptive statistics, including frequencies, means, and standard deviations, were calculated to summarize demographic characteristics and responses. Chi-square tests were performed to evaluate associations between categorical variables, particularly comparing responses between urban and rural livestock producers. A p-value of less than 0.05 was considered statistically significant, indicating meaningful differences in practices and knowledge levels.
2.7. Ethical Considerations
Ethical approval for the study was obtained from the Oromia Agricultural Research Institute. Informed consent was secured from all participants before data collection, ensuring they understood the purpose of the study and their rights to withdraw at any time without consequence. Confidentiality was maintained by anonymizing the data collected.
3. Results
3.1. Demographic Information
A total of 209 respondents participated in the study. The demographic characteristics of the respondents are summarized in
Table 1. Among the respondents, the majority were male, accounting for 150 individuals (71.8%), while females constituted 59 individuals (28.2%). The age of respondents ranged from a minimum of 25 years to a maximum of 65 years. The mean age was 37.96 years with a standard deviation of ± 8.873, indicating a relatively youthful population engaged in livestock farming. The educational backgrounds of the respondents varied significantly, as detailed in
Table 1. The largest proportion of respondents were illiterate (33.5%, n = 70), followed by those who had completed elementary school (30.6%, n = 64). Junior high school graduates represented 18.7% (n = 39), while high school graduates comprised 13.4% (n = 28). Only a small fraction of respondents had pursued higher education, with 2.39% (n = 5) having attended college and 1.44% (n = 3) achieving a university degree.
Table 1. Demographic Information of Respondents.
Demographic Component | Response N (%) |
Sex | |
Male | 150 (71.8) |
Female | 59 (28.2) |
Age | |
Minimum | 25 |
Maximum | 65 |
Mean ± SD | 37.96 ± 8.873 |
Educational Level | |
Illiterate | 70 (33.5) |
Elementary School | 64 (30.6) |
Junior | 39 (18.7) |
High School | 28 (13.4) |
College | 5 (2.39) |
University | 3 (1.44) |
3.2. Veterinary Drug Use and Residue Awareness
The results of the survey reveal varying awareness and practices regarding veterinary drug residue risks among urban and rural respondents. A higher proportion of urban respondents (36.8%) reported awareness of drug residue risks compared to rural respondents (29.8%), though the difference was not statistically significant (p = 0.936). Regarding adherence to withdrawal periods, urban respondents were more likely to follow these guidelines (29.4%) than rural respondents were (22.8%), showing a significant difference (p = 0.018). In terms of reducing drug residues, a similar proportion of urban (28.7%) and rural (24.6%) respondents used boiling as a method, with no significant difference (p = 0.759). However, a larger percentage of rural respondents (44.6%) indicated no action was taken to reduce residues compared to urban respondents (39.5%), although this difference was not statistically significant (p = 0.642).
Table 2. Veterinary Drug Residue Awareness and Practices.
Response Parameter | Urban N (%) | Rural N (%) | p-value |
Aware of Drug Residue Risks | | | |
No | 60 (63.1) | 80 (70.2) | 0.936 |
Yes | 34 (36.8) | 34 (29.8) | |
Followed Withdrawal Period | 28 (29.4) | 26 (22.8) | 0.018 |
Used Boiling to Reduce Residue | 60 (28.7) | 50 (24.6) | 0.759 |
No Action to Reduce Residue | 78 (39.5) | 89 (44.6) | 0.642 |
3.3. Practices Regarding Veterinary Drug Residue Control
The current study results indicate various practices related to veterinary drug residue. A majority of respondents (53.6%) were aware of residues in animal products. However, only 25.8% followed withdrawal periods when using veterinary drugs. A smaller proportion of respondents (18.7%) informed customers about drug use. Additionally, 32.0% reported selling animals after recovery, while 11.5% suspected milk as a potential source of drug residues. These findings highlight areas where practices related to drug residues could be improved.
Table 3. Practices Regarding Veterinary Drug Residue control.
Parameter | Response N (%) |
Aware of Residue in Animal Products | 112 (53.6) |
Followed Withdrawal Period | 54 (25.8) |
Informed Customers About Drug Use | 39 (18.7) |
Sold Animals After Recovery | 67 (32.06) |
Suspected Milk as a Source of Residue | 24 (11.5) |
4. Discussion
4.1. Veterinary Drug Use and Residue Awareness
The study revealed low awareness of the risks associated with veterinary drug residues, with urban respondents displaying slightly higher awareness than rural ones. Similar findings have been noted in Ethiopia, where farmers’ lack of awareness about drug withdrawal periods and residues remains a critical issue. Research in Amhara highlights that only 13.95% of farmers were aware of these risks, emphasizing the need for targeted education initiatives in rural areas to address the knowledge gap
| [9] | MDPI. "Educational interventions for improving livestock farming practices." International Journal of Environmental Research and Public Health, 2020. |
[9]
.
Moreover, our results are consistent with findings from Abebe
et al. | [1] | Abebe, T., et al. (2020). "Urban populations and access to educational resources: Comparative health awareness between rural and urban regions." Journal of Public Health Education. |
[1]
, who reported that urban populations have better access to educational resources and information, leading to higher awareness of health risks compared to rural populations. Similarly, our study reinforces this notion, showing that urban respondents demonstrated greater awareness, with 36.8% acknowledging the risks, compared to 29.8% among rural respondents.
4.2. Withdrawal Periods and Food Safety Practices
The current study regarding the low adherence to withdrawal periods among livestock producers is consistent with existing research in veterinary medicine, particularly in low- and middle-income countries. Adherence to withdrawal periods is crucial for ensuring that animal-derived food products are free from harmful drug residues, thereby safeguarding public health.
A study assessing the knowledge, attitude, and practice of poultry farmers in Zaria highlighted significant gaps in observing withdrawal periods for veterinary drugs in chickens and eggs. Many farmers were unaware of the importance of these periods, increasing the risk of drug residues in food products
| [7] | Kools, J., et al. (2008). "Knowledge and practices of withdrawal periods among livestock farmers." Food Safety Journal. |
[7]
.
In Ethiopia, challenges in veterinary pharmaceutical warehouse management have been documented, indicating systemic issues that may contribute to improper drug use and non-compliance with withdrawal periods. A study focusing on government veterinary clinics and private veterinary drug wholesalers revealed deficiencies in stock management, storage conditions, and regulatory oversight
| [9] | MDPI. "Educational interventions for improving livestock farming practices." International Journal of Environmental Research and Public Health, 2020. |
[9]
.
Furthermore, research on livestock producers' knowledge, attitude, and behavior concerning antimicrobial use and resistance in Ethiopia found that a significant portion of farmers lacked awareness about drug residues and the importance of adhering to withdrawal periods. This lack of knowledge underscores the need for educational interventions to promote responsible drug use among livestock producers
| [12] | ResearchGate. "Knowledge and practices of dairy farmers regarding drug residues." Agricultural Science Journal. Retrieved from https://www.researchgate.net |
[12]
.
The weak enforcement of veterinary drug regulations in Ethiopia has been highlighted as a contributing factor to these challenges. Studies have noted that veterinary drugs and biological products are not effectively regulated and managed in terms of quality, safety, and efficacy, leading to irrational use of medicines
| [1] | Abebe, T., et al. (2020). "Urban populations and access to educational resources: Comparative health awareness between rural and urban regions." Journal of Public Health Education. |
[1]
.
These findings collectively emphasize the necessity for robust regulatory frameworks, effective enforcement mechanisms, and comprehensive education programs to improve compliance with withdrawal periods. Addressing these issues is essential to ensure food safety and public health, particularly in regions where veterinary drug administration practices are suboptimal.
Your observation that a significant proportion of dairy farmers (66.5%) are uncertain about preventing residues in milk aligns with findings from various studies highlighting knowledge gaps in safe milk production practices. Such gaps pose risks to consumer health and threaten the economic viability of dairy farming due to potential market restrictions stemming from non-compliance with food safety standards.
4.3. Consumer Awareness and Health Risks
A study assessing consumers' knowledge regarding antibiotic residues in livestock products found that about 71% had a low level of knowledge, indicating a substantial portion of consumers were unaware of the potential health hazards posed by these residues
| [6] | ICAR E-PUBS. "Consumer knowledge regarding antibiotic residues in livestock products." Indian Council of Agricultural Research, 2022. Retrieved from https://epubs.icar.org.in |
[6]
. This lack of awareness underscores the importance of transparency and communication between producers and consumers to ensure food safety.
4.4. Impact on Consumer Trust
The low percentage of farmers communicating treatment history aligns with findings from other regions, where farmers often neglect to inform consumers about drug usage, thereby diminishing consumer trust and potentially leading to health issues
| [8] | Kumar, A., et al. (2021). "Access to veterinary services and drug use compliance: Lessons from rural farming." Journal of Veterinary Medicine. |
| [11] | PubMed Central. "Antimicrobial use in animal husbandry: A consumer perspective." Journal of Antimicrobial Research. Retrieved from https://pubmed.ncbi.nlm.nih.gov |
[8, 11]
. A study on consumer perceptions of antimicrobial use in animal husbandry highlighted that increased consumer awareness places pressure on animal husbandry to adopt policies to reduce or eliminate antimicrobial use, emphasizing the need for transparency to maintain consumer trust
| [11] | PubMed Central. "Antimicrobial use in animal husbandry: A consumer perspective." Journal of Antimicrobial Research. Retrieved from https://pubmed.ncbi.nlm.nih.gov |
| [13] | Schar, D., et al. (2018). "Educational interventions in livestock farming: Addressing antimicrobial resistance." One Health Journal. |
[11, 13]
.
4.5. Need for Improved Communication Strategies
An integrated review of the role of communication in veterinary settings emphasized that effective communication strategies are essential for promoting responsible antibiotic use and enhancing transparency between farmers, veterinarians, and consumers. Improving communication can lead to better compliance with drug use guidelines and increased consumer confidence in animal-derived food products.
4.6. Conclusion and Recommendations
The findings of this study demonstrate that veterinary drug residues present a significant food safety challenge in Oromia, Ethiopia. To address these challenges, concerted efforts are needed at multiple levels—policy, education, veterinary service provision, and consumer awareness. The following recommendations are offered:
1) Strengthen Regulatory Frameworks: Ethiopia must implement and enforce stricter regulations governing the sale and use of veterinary drugs, particularly regarding the adherence to withdrawal periods.
2) Education and Awareness Campaigns: Educational programs should be developed to increase awareness among livestock producers and consumers about the risks associated with veterinary drug residues and the importance of observing withdrawal periods.
3) Improve Access to Veterinary Services: Rural areas require more accessible veterinary services, including mobile clinics and trained veterinary personnel to provide guidance on proper drug use.
4) Residue Monitoring: Establishment of a national residue monitoring program could help ensure that animal products are screened for veterinary drug residues before reaching consumers.
Abbreviations
CDC | Centers for Disease Control and Prevention |
EFA | Exploratory Factor Analysis |
FAO | Food and Agriculture Organization |
ICAR | Indian Council of Agricultural Research |
KAP | Knowledge, Attitudes, and Practices |
MRLs | Maximum Residue Limits |
SD | Standard Deviation |
SPSS | Statistical Package for the Social Sciences |
WHO | World Health Organization |
Conflicts of Interest
The authors declare no conflicts of interest.
References
| [1] |
Abebe, T., et al. (2020). "Urban populations and access to educational resources: Comparative health awareness between rural and urban regions." Journal of Public Health Education.
|
| [2] |
Bekele, T., et al. (2018). "Non-compliance with withdrawal periods and the occurrence of antimicrobial residues in food animals in Ethiopia." Veterinary World, 11(7), 902-908.
https://doi.org/10.14202/vetworld.2018.902-908
|
| [3] |
Beyene, T. (2016). "Veterinary drug residue management and food safety in Ethiopia: Challenges and opportunities." International Journal of Veterinary Science
|
| [4] |
Beyene, T., & Tesega, B. (2014). "Rational veterinary drug use: Its significance in food safety, public health, and animal productivity." Journal of Veterinary Medicine and Animal Health, 6(12), 359-365.
https://doi.org/10.5897/JVMAH2014.0332
|
| [5] |
Food Science of Animal Resources. (2019). "Thermal stability of quinolones and sulfonamides in food products and implications for consumer safety." Food Science of Animal Resources, 39(6), e58.
https://doi.org/10.5851/kosfa.2019.e58
|
| [6] |
ICAR E-PUBS. "Consumer knowledge regarding antibiotic residues in livestock products." Indian Council of Agricultural Research, 2022. Retrieved from
https://epubs.icar.org.in
|
| [7] |
Kools, J., et al. (2008). "Knowledge and practices of withdrawal periods among livestock farmers." Food Safety Journal.
|
| [8] |
Kumar, A., et al. (2021). "Access to veterinary services and drug use compliance: Lessons from rural farming." Journal of Veterinary Medicine.
|
| [9] |
MDPI. "Educational interventions for improving livestock farming practices." International Journal of Environmental Research and Public Health, 2020.
|
| [10] |
Parmar, K., et al. (2021). "Ensuring food safety by monitoring antimicrobial residues in livestock products." Veterinary World, 14(7), 1650-1664.
https://doi.org/10.14202/vetworld.2021.1650-1664
|
| [11] |
PubMed Central. "Antimicrobial use in animal husbandry: A consumer perspective." Journal of Antimicrobial Research. Retrieved from
https://pubmed.ncbi.nlm.nih.gov
|
| [12] |
ResearchGate. "Knowledge and practices of dairy farmers regarding drug residues." Agricultural Science Journal. Retrieved from
https://www.researchgate.net
|
| [13] |
Schar, D., et al. (2018). "Educational interventions in livestock farming: Addressing antimicrobial resistance." One Health Journal.
|
| [14] |
Treiber, F. M., & Beranek-Knauer, H. (2021). "Antibiotics and food safety: Residues of tetracyclines, sulfonamides, and fluoroquinolones in food products." Antibiotics, 10(5), 534.
https://doi.org/10.3390/antibiotics10050534
|
| [15] |
Wu, Q., et al. (2023). "Advancements in residue detection technologies and strategies for ensuring compliance with veterinary drug regulations." Sustainability, 15(13), 10413.
|
Cite This Article
-
APA Style
Edao, A., Aredo, T. A., Nigatu, Y., Ishetu, S., Arega, A. (2025). Food Safety Concerns Due to Veterinary Drug Residue in Animal Products in Selected Areas in Oromia, Ethiopia. Animal and Veterinary Sciences, 13(1), 1-6. https://doi.org/10.11648/j.avs.20251301.11
 Copy
|
Copy
|
 Download
Download
ACS Style
Edao, A.; Aredo, T. A.; Nigatu, Y.; Ishetu, S.; Arega, A. Food Safety Concerns Due to Veterinary Drug Residue in Animal Products in Selected Areas in Oromia, Ethiopia. Anim. Vet. Sci. 2025, 13(1), 1-6. doi: 10.11648/j.avs.20251301.11
 Copy
|
Copy
|
 Download
Download
AMA Style
Edao A, Aredo TA, Nigatu Y, Ishetu S, Arega A. Food Safety Concerns Due to Veterinary Drug Residue in Animal Products in Selected Areas in Oromia, Ethiopia. Anim Vet Sci. 2025;13(1):1-6. doi: 10.11648/j.avs.20251301.11
 Copy
|
Copy
|
 Download
Download
-
@article{10.11648/j.avs.20251301.11,
author = {Abdela Edao and Tesfaye Alemu Aredo and Yadeta Nigatu and Sisay Ishetu and Alemayehu Arega},
title = {Food Safety Concerns Due to Veterinary Drug Residue in Animal Products in Selected Areas in Oromia, Ethiopia
},
journal = {Animal and Veterinary Sciences},
volume = {13},
number = {1},
pages = {1-6},
doi = {10.11648/j.avs.20251301.11},
url = {https://doi.org/10.11648/j.avs.20251301.11},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.avs.20251301.11},
abstract = {This study investigates the public health risks associated with veterinary drug residues in animal products within selected areas of Oromia, Ethiopia, a region heavily reliant on livestock for economic sustenance and food security. Utilizing a cross-sectional survey methodology, the research involved 209 livestock producers from both urban and rural settings to evaluate their awareness regarding the presence and risks of drug residues in food products, as well as their adherence to prescribed withdrawal periods following veterinary drug administration. The findings reveal alarming knowledge gaps, with a significant portion of respondents (63.1% to 70.2%) unaware of the potential health hazards linked to drug residues in animal-derived food products. Additionally, only 25.8% of farmers reported that they consider withdrawal periods before marketing their livestock or livestock products, indicating a critical lapse in food safety practices. These lapses not only jeopardize public health but also threaten the integrity of the livestock sector and its contribution to the economy. Given these findings, the study underscores the urgent need for improved regulatory frameworks, enhanced educational outreach, and increased access to veterinary services. By implementing targeted interventions aimed at raising awareness and compliance with withdrawal periods, stakeholders can significantly mitigate the risks associated with veterinary drug residues. This research highlights the importance of collaborative efforts among government bodies, veterinary professionals, and livestock producers to ensure safer animal husbandry practices and protect public health in Oromia, Ethiopia.
},
year = {2025}
}
 Copy
|
Copy
|
 Download
Download
-
TY - JOUR
T1 - Food Safety Concerns Due to Veterinary Drug Residue in Animal Products in Selected Areas in Oromia, Ethiopia
AU - Abdela Edao
AU - Tesfaye Alemu Aredo
AU - Yadeta Nigatu
AU - Sisay Ishetu
AU - Alemayehu Arega
Y1 - 2025/01/09
PY - 2025
N1 - https://doi.org/10.11648/j.avs.20251301.11
DO - 10.11648/j.avs.20251301.11
T2 - Animal and Veterinary Sciences
JF - Animal and Veterinary Sciences
JO - Animal and Veterinary Sciences
SP - 1
EP - 6
PB - Science Publishing Group
SN - 2328-5850
UR - https://doi.org/10.11648/j.avs.20251301.11
AB - This study investigates the public health risks associated with veterinary drug residues in animal products within selected areas of Oromia, Ethiopia, a region heavily reliant on livestock for economic sustenance and food security. Utilizing a cross-sectional survey methodology, the research involved 209 livestock producers from both urban and rural settings to evaluate their awareness regarding the presence and risks of drug residues in food products, as well as their adherence to prescribed withdrawal periods following veterinary drug administration. The findings reveal alarming knowledge gaps, with a significant portion of respondents (63.1% to 70.2%) unaware of the potential health hazards linked to drug residues in animal-derived food products. Additionally, only 25.8% of farmers reported that they consider withdrawal periods before marketing their livestock or livestock products, indicating a critical lapse in food safety practices. These lapses not only jeopardize public health but also threaten the integrity of the livestock sector and its contribution to the economy. Given these findings, the study underscores the urgent need for improved regulatory frameworks, enhanced educational outreach, and increased access to veterinary services. By implementing targeted interventions aimed at raising awareness and compliance with withdrawal periods, stakeholders can significantly mitigate the risks associated with veterinary drug residues. This research highlights the importance of collaborative efforts among government bodies, veterinary professionals, and livestock producers to ensure safer animal husbandry practices and protect public health in Oromia, Ethiopia.
VL - 13
IS - 1
ER -
 Copy
|
Copy
|
 Download
Download